Exporting Cosmetics to the U.S.: Essential Regulatory Checklist
With the increasing prosperity of global trade, cosmetic manufacturers and exporters are more frequently expanding their target markets overseas. The United States, as one of the largest economies in the world, is undoubtedly a very attractive market. However, entering the U.S. market is not easy and requires strict compliance with U.S. cosmetic regulations. The goal of this article is to provide a comprehensive guide for those who wish to export cosmetics to the United States.
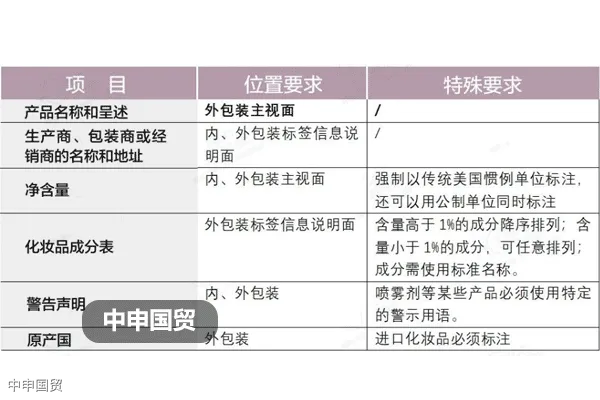













 PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912



