- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
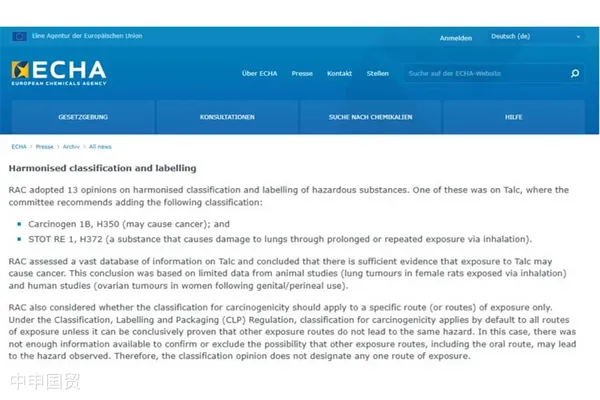
Recently, the European Chemicals Agency (ECHA) announced 13 opinions on the harmonized classification and labeling of hazardous substances adopted by its Risk Assessment Committee (RAC), one of which concerns the classification of talc. The committee unanimously agreed to classify talc as a presumed carcinogen, which under the CLP Regulation should be categorized as a Category 1B carcinogen, applicable to all exposure routes.
In addition, RAC also unanimously agreed to classify talc as specific target organ toxicity (STOT RE 1), concluding that long-term or repeated inhalation of talc can cause lung damage. Therefore, ECHA ultimately proposed the following harmonized classifications for talc:
- Carcinogen 1B, H350
- STOT RE 1, H372
This is undoubtedly a significant change for industries using talc, especiallyCosmetics & Personal Care, enterprises in fields such as pharmaceuticals and food will need to understand the impact of the new regulations as soon as possible and take appropriate measures.
Talc: Diverse Uses and Potential Risks
Talc is a layered mineral composed of magnesium, silicon, and oxygen, with the main component being magnesium silicate, typically appearing as a white or grayish-white powder. Talcs unique lamellar structure gives it a very smooth texture and extremely low hardness, along with good fire resistance, acid resistance, insulation, and adsorption properties.
Talc is widely used in various industries, such as chemicals, healthcare, food, and cosmetics. It serves as a filler in rubber, a bulking agent in tablets, an anti-caking agent in food, and is found in common products like baby powder. However, due to its diverse functions and wide range of uses, recent discussions on its safety (particularly its association with asbestos contamination) have attracted significant international attention.
Scientific Basis for Hazard Classification
In July 2024, the International Agency for Research on Cancer (IARC), a subsidiary of the World Health Organization (WHO), published an article in The Lancet Oncology stating that based on limited human ovarian cancer data, sufficient animal experimental data, and strong mechanistic evidence, talc is classified as a substance possibly carcinogenic to humans.
ECHAs current classification assessment of talc stems from a 2021 classification proposal submitted by the Netherlands (Carc. 2; STOT RE 1). After the formal proposal was submitted, ECHA conducted a comprehensive evaluation of different exposure routes in humans and animals and various types of cancer data, ultimately concluding that there is a potential association between talc and cancer, particularly with lung and ovarian cancer following inhalation and physical contact.
Therefore, ECHA presumes that talc may have carcinogenic effects on humans and recommends classifying it as a Category 1B carcinogen under the CLP Regulation. Although this conclusion still requires final confirmation by the European Commission, ECHAs current assessment sets the tone for the classification change and regulatory direction of talc.
III. Impact on Enterprises and Response Strategies
Once talc is officially confirmed as a Category 1B carcinogen, it will become a Substance of Very High Concern (SVHC) under the REACH Regulation and will be prohibited in cosmetics. For enterprises in the cosmetics, personal care, and pharmaceutical sectors, this means future product manufacturing and sales will face stricter regulatory requirements.
Industry response: Regarding RACs assessment results, the industry has expressed strong opposition, arguing that the existing evidence does not support such a strict classification. Currently, some industry organizations are preparing to take action to influence the European Commissions decision before it is finalized, aiming to mitigate potential negative impacts on the industry.
Corporate response measures: For enterprises producing talc and related products, here are some recommendations to address potential changes:
- Closely monitor regulatory developments:Relevant enterprises should stay updated on the European Commissions final classification decision on talc and understand the implementation timeline of the new regulations to prepare for adjustments in advance.
- Assess the feasibility of alternative substances:Where possible, actively seek and test safe alternatives to talc to ensure market demand can still be met and compliance requirements fulfilled after the new regulations take effect.
- Strengthen product safety assessments:Conduct more rigorous safety assessments of existing products, especially those with high talc content, to adjust formulations if necessary and reduce risks.
- Maintain communication with the supply chain:Ensure that upstream and downstream supply chain partners are aware of this potential regulatory change and jointly develop response measures to minimize uncertainty caused by delayed information.
Summary
The carcinogenicity of talcum powder has long been a focal point of international attention. With ECHAs new classification proposal for talcum powder, manufacturers of related products need to prepare for stricter regulatory requirements. By monitoring regulatory developments, evaluating alternative materials, and enhancing product safety assessments, companies can respond to these changes more effectively, ensuring compliance with the latest regulatory standards and maintaining market competitiveness.
Related Recommendations
? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912