- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
With the continuous development of the toothpaste market and the diversification of consumer demand, the formulation and implementation of relevant regulations and standards have become increasingly important. Recently,Cosmetics & Personal Carethe registration and filing information service platform was updated, adding a Toothpaste User section, which is undoubtedly a signal of upcoming new regulations for the toothpaste industry. This article will explore the details of these new regulations, their requirements, and how businesses should respond.
I. Updates to the Registration and Filing Platform
Addition of the Toothpaste User Section
The Cosmetics Registration and Filing Information Service Platform has been updated, adding a Toothpaste User section under Enterprise Information Management. This change signals the imminent implementation of new toothpaste regulations, undoubtedly adding another layer of compliance requirements for toothpaste manufacturers and sellers.
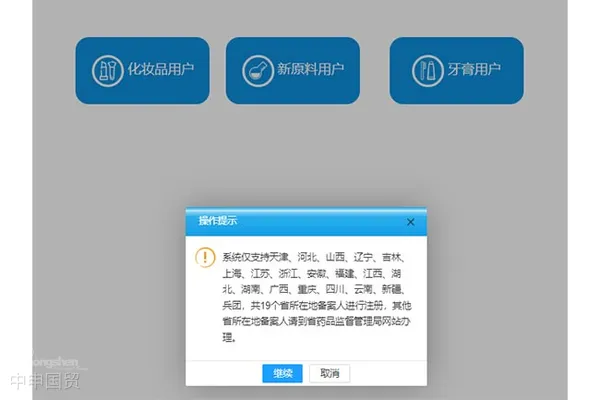
Restrictions on Application Provinces
The newly added Toothpaste User section limits the provinces eligible for filing, with filers from 19 provinces—including Tianjin, Hebei, Shanxi, Liaoning, Jilin, Shanghai, Jiangsu, Zhejiang, Anhui, Fujian, Jiangxi, Hubei, Hunan, Guangxi, Chongqing, Sichuan, Yunnan, Xinjiang, and the Xinjiang Production and Construction Corps—able to register on the platform. Filers from other provinces must complete the process on their respective provincial drug administration websites.
Preparation of Filer Materials
Both domestic and overseas filers must prepare a series of documents for registration, including a filer information form, quality and safety responsible person information, quality management system overview, adverse reaction monitoring system overview, etc. Overseas filers additionally need to provide an authorization letter, manufacturer information form, GMP documents, etc.
II. Published Toothpaste Regulatory Requirements
Toothpaste Supervision and Management Measures
Currently, published toothpaste regulations include the Toothpaste Supervision and Management Measures and the Cosmetics Safety Technical Standards revision project, among others. These regulations provide clear guidance for the production, sale, and use of toothpaste.
Addition of Testing Items
Regarding physicochemical testing items, toothpaste is similar to cosmetics but includes additional tests for hard particles, fluoride content, diethylene glycol, and ethylene glycol. These testing items are stipulated in the national toothpaste standards.
Standardization of Efficacy Claims
For efficacy claims, the Toothpaste Filing Documentation Standards (draft for comments) limits the scope of claims toothpaste can make. Companies should use cautious language in efficacy claims to ensure compliance with regulatory requirements.
III. Toothpaste Regulations Worth Attention
Toothpaste Ingredient Catalog
The Catalog of Used Toothpaste Ingredients will be used to determine whether new ingredients are used and standardize ingredient INCI names and Chinese names. This will help ensure the safety and quality of toothpaste products.
Toothpaste Labeling Management Measures
The Toothpaste Labeling Management Measures will clarify mandatory labeling requirements for toothpaste. It is reported that the measures will be formulated with reference to the Cosmetics Labeling Management Measures, providing clear guidance for toothpaste product labeling.
Toothpaste Safety Assessment Technical Guidelines
The Toothpaste Safety Assessment Technical Guidelines will be used to assess the safety of toothpaste products, providing a scientific basis for their safe use.
IV. Corporate Response Strategies
Prepare Documentation in Advance
With the new regulations set to take effect on December 1, companies have just over two months to prepare. Therefore, companies should prepare the necessary documentation in advance to ensure smooth registration and filing of toothpaste products once the new regulations are implemented.
Closely Monitor Regulatory Developments
Companies must also closely monitor the release and updates of toothpaste-related regulations, staying informed of regulatory requirements to ensure their production and sales activities comply with laws and regulations.
Strengthen Product Quality and Safety Management
Companies should strengthen product quality and safety management to ensure compliance with regulatory requirements and enhance market competitiveness.
Related Recommendations
? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912